Financial Release
"
Second-Quarter Results
- Worldwide net revenues were
$6.45 billion in the second quarter, up 17.9 percent. On an operational basis, adjusted net revenues increased 18.0 percent, excluding a 0.5 percent unfavorable impact from foreign exchange rate fluctuations. - Global HUMIRA sales increased 17.4 percent on a reported basis. Operational HUMIRA sales increased 17.7 percent, excluding a modest impact of foreign exchange. Strong global growth was driven by continued momentum across all three major market categories – rheumatology, dermatology and gastroenterology.
- Second-quarter global IMBRUVICA net revenue was
$439 million , with U.S. sales of$384 million and international profit sharing of$55 million for the quarter. - Total company revenue growth was also driven by
$419 million in global VIEKIRA sales in the quarter, as well as strong operational growth from Duodopa, Creon and Lupron. - On a GAAP basis, the gross margin ratio in the second quarter was 78.2 percent. The adjusted gross margin ratio was 81.9 percent, excluding intangible asset amortization and other specified items.
- On a GAAP basis, selling, general and administrative (SG&A) expense was 22.7 percent of net revenues in the second quarter. The adjusted SG&A expense was 22.2 percent of net revenues.
- On a GAAP basis, research and development (R&D) expense was 17.4 percent of net revenues in the second quarter. The adjusted R&D expense was 15.5 percent, reflecting funding actions supporting all stages of our pipeline and the impact from the Stemcentrx and Boehringer Ingelheim transactions.
- On a GAAP basis, the operating margin in the second quarter was 37.0 percent. The adjusted operating margin was 43.9 percent. The company remains committed to an adjusted operating margin target of greater than 50 percent by 2020.
- Net interest expense was
$225 million . On a GAAP basis, the tax rate in the quarter was 23.2 percent. The adjusted tax rate was 20.1 percent. - Diluted earnings per share (EPS) was
$0.98 on a GAAP basis. Adjusted diluted EPS, excluding intangible asset amortization expense and other specified items, was$1.26 in the second quarter, up 16.7 percent.
Key Events from the Second Quarter
- On
June 1 ,AbbVie successfully completed the acquisition of Stemcentrx and its lead late-stage asset, rovalpituzumab tesirine (Rova-T), further strengthening the company's oncology portfolio by providing a highly attractive platform in solid tumors. Rova-T is a novel biomarker-specific therapy that targets cancer stem cells and combines a targeted antibody that delivers a cytotoxic agent directly to cancer cells expressing delta-like protein 3 (DLL3). DLL is expressed in more than 80 percent of small cell lung cancer (SCLC) patient tumors and is not present in healthy tissue. New data, presented at theAmerican Society of Clinical Oncology (ASCO ) meeting in early June, demonstrated that Rova-T outperformed the best reference for standard of care in both overall response rate (ORR) and 12-month overall survival (OS). A registrational trial for third-line SCLC is expected to complete enrollment by the end of 2016, supporting regulatory submissions in 2017. The expression of DLL3 suggests Rova-T may be useful across multiple tumor types.AbbVie is also advancing studies to evaluate Rova-T in other tumor types, as well as a first-line treatment for SCLC. AbbVie andBristol-Myers Squibb Company recently announced a clinical trial collaboration to evaluate the safety, tolerability and efficacy of Rova-T in combination withBristol-Myers Squibb's OPDIVO (nivolumab) and OPDIVO + YERVOY (ipilimumab) regimen as a second-line treatment for extensive-stage SCLC. This collaboration will explore the potential enhanced efficacy of combining checkpoint inhibitors with an antibody drug conjugate in SCLC and is expected to begin in 2016.AbbVie announced that theEuropean Commission (EC) approved IMBRUVICA (ibrutinib) as a first-line treatment option for patients with chronic lymphocytic leukemia (CLL) inEurope . This approval followed theU.S. Food and Drug Administration (FDA ) approval for the same indication earlier in the year, and represents the fifth treatment indication for IMBRUVICA to date. IMBRUVICA is the first chemotherapy-free treatment option approved for first-line CLL in theEuropean Union (EU).- In June,
AbbVie announced that theFDA granted a fourth Breakthrough Therapy Designation (BTD) for IMBRUVICA as a potential treatment of chronic graft-versus-host-disease (cGvHD) after failure of one or more lines of systemic therapy. cGvHD is a severe and potentially life-threatening condition in which transplanted cells from the donor attack the patient's body. There are currently no approved therapies for this common complication, which patients may develop after undergoing allogeneic stem cell or bone marrow transplantation in which they receive cells from a donor. This BTD was based on clinical data from a Phase 1b/2 study which found that IMBRUVICA demonstrated promising early clinical efficacy data supporting an improvement in cGvHD based on theNational Institutes of Health (NIH) Consensus Response Criteria . - In May, the
FDA updated the IMBRUVICA label to include new data from two Phase 3 trials supporting its expanded use in patients with CLL and small lymphocytic lymphoma (SLL). The label now also includes OS results in treatment-naïve patients with CLL/SLL from the Phase 3 RESONATE-2 trial, as well as safety and efficacy data from the Phase 3 HELIOS trial assessing the use of IMBRUVICA in combination with bendamustine and rituximab (BR) versus placebo plus BR in relapsed/refractory (R/R) patients with CLL/SLL. - In April,
AbbVie received acceleratedFDA approval of VENCLEXTA (venetoclax) for patients with R/R CLL with 17p deletion, a condition which is typically associated with a poor prognosis, and found in up to 30 to 50 percent of these previously-treated patients. New data on venetoclax in patients with acute myeloid leukemia (AML) were presented atASCO as part of the meeting's "Best ofASCO " program. These data found that venetoclax plus hypomethylating agents (HMA) demonstrated a higher ORR of 70 percent and 71 percent at 400 mg and 800 mg, respectively, which is roughly double the response rate that would be expected with current standard of care.AbbVie plans to start a Phase 3 study in AML by year-end. The company also recently initiated a Phase 3 trial to evaluate venetoclax plus standard of care in patients with multiple myeloma (MM). VENCLEXTA is being developed byAbbVie and Genentech, a member of theRoche Group . AbbVie announced theFDA granted ABT-414, an investigational antibody drug conjugate targeting the epidermal growth factor receptor (EGFR), a Rare Pediatric Disease Designation for the treatment of pediatric patients with EGFR-amplified Diffuse Intrinsic Pontine Glioma, known to be a highly aggressive and difficult-to-treat tumor found at the base of the brain. ABT-414 is also being evaluated in Phase 2 studies for the treatment of adult patients with EGFR-amplified glioblastoma multiforme, an aggressive malignant primary brain tumor.Bristol-Myers Squibb ,AbbVie's development and commercialization partner, announced the EC approval of EMPLICITI (elotuzumab) for the treatment of MM as a combination therapy with REVLIMID (lenalidomide) and dexamethasone in adult patients who have received at least one prior therapy. This makes EMPLICITI the first and only immunostimulatory antibody available in the EU for patients with MM. EMPLICITI was approved by theFDA in late 2015.AbbVie and Biogen announced theFDA and EC approvals for ZINBRYTA (daclizumab), a once-monthly, self-administered, subcutaneous treatment for relapsing forms of multiple sclerosis (RMS). These approvals were based on results from the Phase 3 DECIDE and SELECT trials which demonstrated that treatment with ZINBRYTA 150 mg, administered subcutaneously every four weeks, reduced the annualized relapse rate, as well as the risk of 24-week confirmed disability progression. ZINBRYTA improved results on key measures of MS disease activity in patients with RMS compared to AVONEX 30 mcg intramuscular injection administered weekly and placebo. The companies plan to launch ZINBRYTA in the U.S. andGermany in August.AbbVie announced theFDA approval of HUMIRA for the treatment of non-infectious intermediate, posterior and panuveitis in adult patients, a disease that can severely impact vision. HUMIRA is the first and onlyFDA -approved non-corticosteroid therapy available for these patients. This approval marks the 10th approved indication for HUMIRA in the United States and is based on results from two pivotal Phase 3 studies, which demonstrated that patients treated with HUMIRA had a significantly lower risk for uveitic flare or a decrease in visual acuity compared to placebo. TheCommittee for Medicinal Products for Human Use (CHMP) also recently granted a positive opinion for this indication.AbbVie presented Phase 2 data on risankizumab, an anti-IL-23 monoclonal biologic antibody being developed in collaboration with Boehringer Ingelheim, at the annual Digestive Disease Week (DDW) conference. Results demonstrated that in patients with moderate-to-severe Crohn's disease, risankizumab was more effective than placebo. After 12 weeks, 24 percent and 37 percent of patients achieved clinical remission (no symptoms or very mild symptoms of disease) with 200 mg and 600 mg risankizumab, respectively, compared with 15 percent of patients receiving placebo. Endoscopic remission (normalization of the lining of bowel as seen during an endoscopy) was achieved by 15 percent and 20 percent of patients receiving 200 mg and 600 mg risankizumab, respectively, compared with 3 percent of patients receiving placebo. Risankizumab is also in Phase 3 studies for psoriasis and being evaluated in mid-stage trials for psoriatic arthritis.AbbVie presented new data from its investigational chronic hepatitis C virus (HCV) infection development program for ABT-493 and ABT-530, a once-daily, ribavirin (RBV)-free, pan-genotypic regimen at The International Liver Congress™ (EASL). Results demonstrated that 97 to 98 percent of genotype 1-3 (GT1-3) HCV infected patients without cirrhosis treated with the regimen achieved sustained virologic response at 12 weeks post-treatment (SVR12); 100 percent of genotype 4-6 (GT4-6) patients without cirrhosis achieved SVR12 with 12 weeks of treatment; and GT3 patients with compensated cirrhosis (Child-Pugh A), historically considered difficult-to-treat, achieved 100 percent SVR12 with 12 weeks of treatment. Further results fromAbbVie's investigational HCV development program will be presented later in the year and the company anticipates commercialization of the next-generation combination in 2017.AbbVie hosted its R&D Day meeting onJune 3 for members of the investment community and media, highlighting details of the company's innovative pipeline. During the event,AbbVie presented an overview of its pipeline, reinforcing the company's strategy to develop new therapies to significantly advance and reset the standard of care.AbbVie's late-stage pipeline consists of multiple investigational assets that have generated data demonstrating their potential to improve the standard of care. Supporting materials from R&D Day, including the event presentation and an archived webcast, can be found onAbbVie's Investor Relations website at www.abbvieinvestor.com.
AbbVie Raises Full-Year 2016 Outlook
About
Conference Call
Non-GAAP Financial Results
Financial results for 2015 and 2016 are presented on both a reported and a non-GAAP basis. Reported results were prepared in accordance with GAAP and include all revenue and expenses recognized during the period. Non-GAAP results adjust for certain non-cash items and for factors that are unusual or unpredictable, and exclude those costs, expenses, and other specified items presented in the reconciliation tables later in this release.
Forward-Looking Statements
Some statements in this news release are, or may be considered, forward-looking statements for purposes of the Private Securities Litigation Reform Act of 1995. The words "believe," "expect," "anticipate," "project" and similar expressions, among others, generally identify forward-looking statements. AbbVie cautions that these forward-looking statements are subject to risks and uncertainties that may cause actual results to differ materially from those indicated in the forward-looking statements. Such risks and uncertainties include, but are not limited to, challenges to intellectual property, competition from other products, difficulties inherent in the research and development process, adverse litigation or government action, and changes to laws and regulations applicable to our industry. Additional information about the economic, competitive, governmental, technological and other factors that may affect
|
AbbVie Inc. |
||||||||
|
% Change vs. 2Q15 |
||||||||
|
Net Revenues (in millions) |
International |
Total |
||||||
|
U.S. |
Int'l. |
Total |
U.S. |
Operational |
Reported |
Operational |
Reported |
|
|
ADJUSTED NET REVENUES |
$4,100a |
$2,332 |
$6,432a |
21.7%a |
12.1% |
10.8% |
18.0%a |
17.5%a |
|
Humira |
2,712 |
1,437 |
4,149 |
26.7 |
4.0 |
3.0 |
17.7 |
17.4 |
|
Imbruvica |
384 |
55b |
439 |
>100.0 |
>100.0 |
>100.0 |
>100.0 |
>100.0 |
|
Viekira |
87 |
332 |
419 |
(61.4) |
>100.0 |
>100.0 |
8.2 |
9.1 |
|
Lupron |
179 |
40 |
219 |
14.6 |
(1.4) |
(5.3) |
11.2 |
10.4 |
|
Synagis |
-- |
45 |
45 |
n/a |
8.3 |
(2.6) |
8.3 |
(2.6) |
|
Synthroid |
188 |
-- |
188 |
1.1 |
n/a |
n/a |
1.1 |
1.1 |
|
Creon |
180 |
-- |
180 |
12.9 |
n/a |
n/a |
12.9 |
12.9 |
|
AndroGel |
171 |
-- |
171 |
1.0 |
n/a |
n/a |
1.0 |
1.0 |
|
Kaletra |
30 |
116 |
146 |
(30.4) |
1.0 |
(6.0) |
(7.2) |
(12.4) |
|
Sevoflurane |
22 |
92 |
114 |
6.5 |
(1.9) |
(5.8) |
(0.6) |
(3.8) |
|
Duodopa |
9 |
64 |
73 |
>100.0 |
18.2 |
20.8 |
28.7 |
31.2 |
|
Note: "Operational" growth reflects the percentage change over the prior year excluding the impact of exchange rate fluctuations. |
|
n/a = not applicable |
|
a U.S. and total net revenues for the quarter ended June 30, 2016 exclude specified items. Refer to the Reconciliation of GAAP Reported to Non-GAAP Adjusted Information for further details. Percentage change is calculated using adjusted net revenues. |
|
b Reflects profit sharing for Imbruvica international revenues. |
|
AbbVie Inc. |
||||||||
|
% Change vs. 6M15 |
||||||||
|
Net Revenues (in millions) |
International |
Total |
||||||
|
U.S. |
Int'l. |
Total |
U.S. |
Operational |
Reported |
Operational |
Reported |
|
|
ADJUSTED NET REVENUES |
$7,594a |
$4,796 |
$12,390a |
26.2%a |
12.0% |
6.7% |
20.1%a |
17.8%a |
|
Humira |
4,907 |
2,819 |
7,726 |
29.0 |
4.3 |
(0.8) |
18.4 |
16.2 |
|
Imbruvica |
709 |
111 |
820 |
>100.0 |
>100.0 |
>100.0 |
>100.0 |
>100.0 |
|
Viekira |
212 |
621 |
833 |
(41.7) |
>100.0 |
>100.0 |
37.7 |
35.4 |
|
Lupron |
330 |
79 |
409 |
8.0 |
1.0 |
(6.4) |
6.5 |
4.9 |
|
Synagis |
-- |
364 |
364 |
n/a |
2.8 |
(4.4) |
2.8 |
(4.4) |
|
Synthroid |
370 |
-- |
370 |
(0.7) |
n/a |
n/a |
(0.7) |
(0.7) |
|
Creon |
330 |
-- |
330 |
15.2 |
n/a |
n/a |
15.2 |
15.2 |
|
AndroGel |
327 |
-- |
327 |
1.5 |
n/a |
n/a |
1.5 |
1.5 |
|
Kaletra |
63 |
216 |
279 |
(25.2) |
(9.5) |
(17.6) |
(13.3) |
(19.5) |
|
Sevoflurane |
39 |
186 |
225 |
2.7 |
(4.2) |
(9.9) |
(3.1) |
(7.9) |
|
Duodopa |
16 |
125 |
141 |
>100.0 |
20.9 |
18.7 |
32.6 |
30.5 |
|
Note: "Operational" growth reflects the percentage change over the prior year excluding the impact of exchange rate fluctuations. |
|
n/a = not applicable |
|
a U.S. and total net revenues for the six months ended June 30, 2016 exclude specified items. Refer to the Reconciliation of GAAP Reported to Non-GAAP Adjusted Information for further details. Percentage change is calculated using adjusted net revenues. |
|
b Reflects profit sharing for Imbruvica international revenues. |
|
AbbVie Inc. |
||||||||
|
Second Quarter Ended June 30 |
Six Months Ended June 30 |
|||||||
|
2016 |
2015 |
2016 |
2015 |
|||||
|
Net revenues |
$6,452 |
$5,475 |
$12,410 |
$10,515 |
||||
|
Cost of products sold |
1,405 |
916 |
2,774 |
1,858 |
||||
|
Selling, general and administrative |
1,466 |
1,703 |
2,821 |
3,176 |
||||
|
Research and development |
1,124 |
981 |
2,070 |
1,792 |
||||
|
Acquired in-process research and development |
70 |
23 |
80 |
150 |
||||
|
Total operating cost and expenses |
4,065 |
3,623 |
7,745 |
6,976 |
||||
|
Operating earnings |
2,387 |
1,852 |
4,665 |
3,539 |
||||
|
Interest expense, net |
225 |
164 |
425 |
290 |
||||
|
Net foreign exchange loss |
15 |
14 |
317 |
178 |
||||
|
Other expense (income), net |
51 |
(4) |
51 |
(3) |
||||
|
Earnings before income tax expense |
2,096 |
1,678 |
3,872 |
3,074 |
||||
|
Income tax expense |
486 |
312 |
908 |
686 |
||||
|
Net earnings |
$1,610 |
$1,366 |
$2,964 |
$2,388 |
||||
|
Diluted earnings per share |
$0.98 |
$0.83 |
$1.81 |
$1.47 |
||||
|
Diluted earnings per share, excluding specified itemsa |
$1.26 |
$1.08 |
$2.41 |
$2.03 |
||||
|
Weighted-average diluted shares outstanding |
1,632 |
1,633 |
1,629 |
1,621 |
||||
|
a Refer to the Reconciliation of GAAP Reported to Non-GAAP Adjusted Information for further details. |
|
AbbVie Inc. |
|||
|
1. Specified items impacted results as follows: |
|||
|
2Q16 |
|||
|
Earnings |
Diluted |
||
|
Pre-tax |
After-tax |
EPS |
|
|
As reported (GAAP) |
$2,096 |
$1,610 |
$0.98 |
|
Adjusted for specified items: |
|||
|
Intangible asset amortization |
181 |
144 |
0.09 |
|
Milestones and other R&D expenses |
55 |
55 |
0.03 |
|
Acquired IPR&D |
70 |
70 |
0.04 |
|
Acquisition related costs |
145 |
122 |
0.08 |
|
Change in fair value of contingent consideration |
41 |
41 |
0.02 |
|
Other |
4 |
30 |
0.02 |
|
As adjusted (non-GAAP) |
$2,592 |
$2,072 |
$1.26 |
|
Milestones and other R&D expenses are associated with milestone payments for previously announced collaborations. Acquired IPR&D primarily reflects upfront payments related to licensing arrangements with third parties. Acquisition related costs primarily includes compensation expense, financing and other costs associated with the acquisition of Stemcentrx and Boehringer Ingelheim, as well as the amortization of the acquisition date fair value step-up for inventory related to the acquisition of Pharmacyclics. Other includes restructuring charges associated with streamlining global operations, a charge to increase tax reserves, and milestone revenue under a previously announced collaboration. |
|
2. The impact of the specified items by line item was as follows: |
||||||
|
2Q16 |
||||||
|
Net revenues |
Cost of |
SG&A |
R&D |
Acquired IPR&D |
Other expense, net |
|
|
As reported (GAAP) |
$6,452 |
$1,405 |
$1,466 |
$1,124 |
$70 |
$51 |
|
Adjusted for specified items: |
||||||
|
Intangible asset amortization |
-- |
(181) |
-- |
-- |
-- |
-- |
|
Milestones and other R&D expenses |
-- |
-- |
-- |
(55) |
-- |
-- |
|
Acquired IPR&D |
-- |
-- |
-- |
-- |
(70) |
-- |
|
Acquisition related costs |
-- |
(46) |
(15) |
(72) |
-- |
(12) |
|
Change in fair value of contingent consideration |
-- |
-- |
-- |
-- |
-- |
(41) |
|
Other |
(20) |
(9) |
(15) |
-- |
-- |
-- |
|
As adjusted (non-GAAP) |
$6,432 |
$1,169 |
$1,436 |
$997 |
$-- |
($2) |
|
3. The adjusted tax rate for the second quarter of 2016 was 20.1 percent, as detailed below: |
|||
|
2Q16 |
|||
|
Pre-tax |
Income |
||
|
income |
taxes |
Tax rate |
|
|
As reported (GAAP) |
$2,096 |
$486 |
23.2% |
|
Specified items |
496 |
34 |
6.9% |
|
As adjusted (non-GAAP) |
$2,592 |
$520 |
20.1% |
|
AbbVie Inc. |
|||
|
1. Specified items impacted results as follows: |
|||
|
2Q15 |
|||
|
Earnings |
Diluted |
||
|
Pre-tax |
After-tax |
EPS |
|
|
As reported (GAAP) |
$1,678 |
$1,366 |
$0.83 |
|
Adjusted for specified items: |
|||
|
Intangible asset amortization |
86 |
66 |
0.04 |
|
Separation costs |
95 |
80 |
0.05 |
|
Acquired IPR&D |
23 |
23 |
0.01 |
|
Pharmacyclics acquisition related costs |
359 |
215 |
0.13 |
|
Other |
34 |
26 |
0.02 |
|
As adjusted (non-GAAP) |
$2,275 |
$1,776 |
$1.08 |
|
Separation costs are expenses related to the separation of AbbVie from Abbott. Acquired IPR&D primarily reflects an upfront payment related to a licensing arrangement with a third party. Pharmacyclics acquisition related costs reflect compensation expense, transaction, financing, integration and other costs related to the acquisition of Pharmacyclics. Other is primarily associated with restructuring activities. |
|
2. The impact of the specified items by line item was as follows: |
|||||
|
2Q15 |
|||||
|
Cost of |
SG&A |
R&D |
Acquired IPR&D |
Interest |
|
|
As reported (GAAP) |
$916 |
$1,703 |
$981 |
$23 |
$164 |
|
Adjusted for specified items: |
|||||
|
Intangible asset amortization |
(86) |
-- |
-- |
-- |
-- |
|
Separation costs |
(2) |
(93) |
-- |
-- |
-- |
|
Acquired IPR&D |
-- |
-- |
-- |
(23) |
-- |
|
Pharmacyclics acquisition related costs |
(19) |
(220) |
(93) |
-- |
(27) |
|
Other |
(3) |
(15) |
(16) |
-- |
-- |
|
As adjusted (non-GAAP) |
$806 |
$1,375 |
$872 |
-- |
$137 |
|
3. The adjusted tax rate for the second quarter of 2015 was 21.9 percent, as detailed below: |
|||
|
2Q15 |
|||
|
Pre-tax |
Income |
||
|
income |
taxes |
Tax rate |
|
|
As reported (GAAP) |
$1,678 |
$312 |
18.6% |
|
Specified items |
597 |
187 |
31.3% |
|
As adjusted (non-GAAP) |
$2,275 |
$499 |
21.9% |
|
AbbVie Inc. |
|||
|
1. Specified items impacted results as follows: |
|||
|
6M16 |
|||
|
Earnings |
Diluted |
||
|
Pre-tax |
After-tax |
EPS |
|
|
As reported (GAAP) |
$3,872 |
$2,964 |
$1.81 |
|
Adjusted for specified items: |
|||
|
Intangible asset amortization |
346 |
277 |
0.17 |
|
Milestones and other R&D expenses |
70 |
70 |
0.04 |
|
Acquired IPR&D |
80 |
80 |
0.05 |
|
Acquisition related costs |
204 |
159 |
0.11 |
|
Change in fair value of contingent consideration |
41 |
41 |
0.02 |
|
Foreign exchange loss |
298 |
298 |
0.18 |
|
Other |
44 |
57 |
0.03 |
|
As adjusted (non-GAAP) |
$4,955 |
$3,946 |
$2.41 |
|
Milestones and other R&D expenses are associated with milestone payments for previously announced collaborations. Acquired IPR&D primarily reflects upfront payments related to licensing arrangements with third parties. Acquisition related costs primarily includes compensation expense, financing and other costs associated with the acquisition of Stemcentrx and Boehringer Ingelheim, as well as the amortization of the acquisition date fair value step-up for inventory related to the acquisition of Pharmacyclics. The foreign exchange loss relates to a devaluation of AbbVie's net monetary assets denominated in the Venezuelan bolivar. Other includes a charge for the impairment of an intangible asset, restructuring charges associated with streamlining global operations, a charge to increase tax reserves, and milestone revenue under a previously announced collaboration. |
|
2. The impact of the specified items by line item was as follows: |
|||||||
|
6M16 |
|||||||
|
Net revenues |
Cost of |
SG&A |
R&D |
Acquired IPR&D |
Net foreign |
Other |
|
|
As reported (GAAP) |
$12,410 |
$2,774 |
$2,821 |
$2,070 |
$80 |
$317 |
$51 |
|
Adjusted for specified items: |
|||||||
|
Intangible asset amortization |
-- |
(346) |
-- |
-- |
-- |
-- |
-- |
|
Milestones and other R&D expenses |
-- |
-- |
-- |
(70) |
-- |
-- |
-- |
|
Acquired IPR&D |
-- |
-- |
-- |
-- |
(80) |
-- |
-- |
|
Acquisition related costs |
-- |
(91) |
(20) |
(81) |
-- |
-- |
(12) |
|
Change in fair value of contingent consideration |
-- |
-- |
-- |
-- |
-- |
-- |
(41) |
|
Venezuela devaluation loss |
-- |
-- |
-- |
-- |
-- |
(298) |
-- |
|
Other |
(20) |
(53) |
(18) |
7 |
-- |
-- |
-- |
|
As adjusted (non-GAAP) |
$12,390 |
$2,284 |
$2,783 |
$1,926 |
$-- |
$19 |
($2) |
|
3. The adjusted tax rate for the first half of 2016 was 20.4 percent, as detailed below: |
|||
|
6M16 |
|||
|
Pre-tax |
Income |
||
|
income |
taxes |
Tax rate |
|
|
As reported (GAAP) |
$3,872 |
$908 |
23.4% |
|
Specified items |
1,083 |
101 |
9.4% |
|
As adjusted (non-GAAP) |
$4,955 |
$1,009 |
20.4% |
|
AbbVie Inc. |
|||
|
1. Specified items impacted results as follows: |
|||
|
6M15 |
|||
|
Earnings |
Diluted |
||
|
Pre-tax |
After-tax |
EPS |
|
|
As reported (GAAP) |
$3,074 |
$2,388 |
$1.47 |
|
Adjusted for specified items: |
|||
|
Intangible asset amortization |
154 |
118 |
0.07 |
|
Separation costs |
199 |
169 |
0.10 |
|
Acquired IPR&D |
150 |
150 |
0.09 |
|
Pharmacyclics acquisition related costs |
420 |
256 |
0.16 |
|
Shire termination |
170 |
170 |
0.10 |
|
Other |
68 |
49 |
0.04 |
|
As adjusted (non-GAAP) |
$4,235 |
$3,300 |
$2.03 |
|
Separation costs are expenses related to the separation of AbbVie from Abbott. Acquired IPR&D primarily reflects the C2N collaboration. Pharmacyclics acquisition related costs reflect compensation expense, transaction, financing, integration and other costs related to the acquisition of Pharmacyclics. Shire termination reflects the completed liquidation of remaining foreign currency positions related to the terminated Shire transaction. Other is primarily associated with restructuring activities. |
|
2. The impact of the specified items by line item was as follows: |
||||||
|
6M15 |
||||||
|
Cost of products sold |
SG&A |
R&D |
Acquired IPR&D |
Interest expense, net |
Net foreign exchange loss |
|
|
As reported (GAAP) |
$1,858 |
$3,176 |
$1,792 |
$150 |
$290 |
$178 |
|
Adjusted for specified items: |
||||||
|
Intangible asset amortization |
(154) |
-- |
-- |
-- |
-- |
-- |
|
Separation costs |
(5) |
(194) |
-- |
-- |
-- |
-- |
|
Acquired IPR&D |
-- |
-- |
-- |
(150) |
-- |
-- |
|
Pharmacyclics acquisition related costs |
(19) |
(222) |
(93) |
-- |
(86) |
-- |
|
Shire termination |
-- |
-- |
-- |
-- |
-- |
(170) |
|
Other |
(12) |
(40) |
(16) |
-- |
-- |
-- |
|
As adjusted (non-GAAP) |
$1,668 |
$2,720 |
$1,683 |
-- |
$204 |
$8 |
|
3. The adjusted tax rate for the first half of 2015 was 22.1 percent, as detailed below: |
|||
|
6M15 |
|||
|
Pre-tax |
Income |
||
|
income |
taxes |
Tax rate |
|
|
As reported (GAAP) |
$3,074 |
$686 |
22.3% |
|
Specified items |
1,161 |
249 |
21.4% |
|
As adjusted (non-GAAP) |
$4,235 |
$935 |
22.1% |
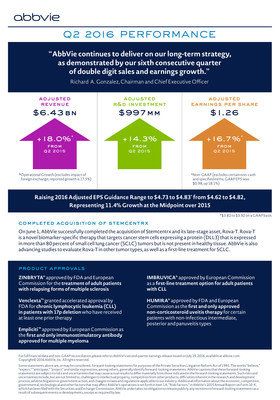

Photo - http://photos.prnewswire.com/prnh/20160728/394051-INFO
Logo - http://photos.prnewswire.com/prnh/20160706/386913LOGO
To view the original version on PR Newswire, visit:http://www.prnewswire.com/news-releases/abbvie-reports-second-quarter-2016-financial-results-300306184.html
SOURCE
Media: Adelle Infante, (847) 938-8745; Investors: Liz Shea, (847) 935-2211; Sharon Greenlees, (847) 935-0900; Todd Bosse, (847) 936-1182
